Abstract
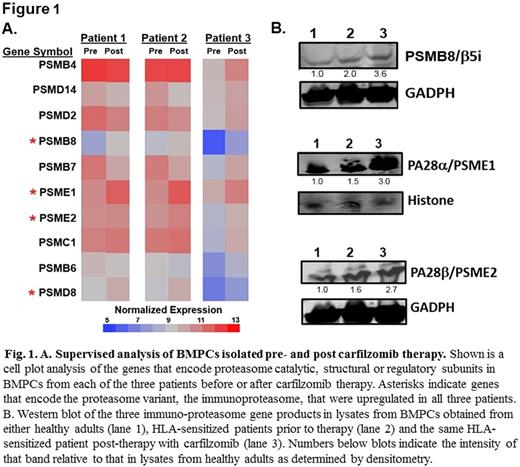
Bone marrow plasma cells (BMPCs) are the source of long-term humoral immunity including HLA antibodies, which promote organ rejection. End-stage renal disease (ESRD) affects more than 660,000 patients per year in the U.S. and is associated with significant morbidity and mortality. Renal transplantation remains the optimal treatment for a substantial proportion of ESRD patients and dramatically improves patient overall survival and quality-of-life. However, preformed human leukocyte antigen (HLA)-specific antibodies are associated with reduced transplantation rates, graft loss and poorer outcomes. Proteasome inhibitor (PI) therapies that target BMPCs to reduce HLA-antibody levels represent an effective means of enhancing transplantation outcomes but are limited by resistance mechanisms. Single antigen bead assays determined that the spectrum of antibody production by HLA-sensitized patient (BMPCs) was remarkably similar to that detected in matching serum samples, supporting the role of BMPCs as the primary source of circulating HLA antibodies. Desensitization therapy with the irreversible proteasome inhibitor carfilzomib reduced PCs in patient BM by ~70% and was associated with a profound reduction in HLA antibodies. We then used bulk RNA-seq transcriptomics to explore potential drug resistance mechanisms within carfilzomib-resistant BMPCs. Network-based pathway analysis revealed that genes encoding the immunoproteasome, a specialized proteasome variant with unique catalytic subunits, and the proteasome activator PA28 were upregulated in BMPCs that survived carfilzomib therapy. Native gel electrophoresis and in vitro functional studies confirmed that BMPCs isolated after carfilzomib therapy contained greater levels of immunoproteasomes as well as increased immunoproteasome-specific peptide-hydrolyzing activity relative to BMPCs from healthy donors or untreated HLA-sensitized patients. Supervised hierarchical clustering analysis (Fig. 1A) and western blotting (Fig. 1B) indicated that PSMB8, which encodes an immunoproteasome catalytic subunit, and PSME1 and PSME2 which encode the proteasome PA28 activator, were significantly upregulated in BMPCs isolated from patients following carfilzomib therapy. Assays then demonstrated that BMPCs that survived carfilzomib therapy were resistant to the FDA-approved PIs bortezomib, carfilzomib and ixazomib but were sensitive to an immunoproteasome-specific inhibitor KZR-616. Thus, we have uncovered proteasomal adaptations in BMPCs as a resistance mechanism underlying carfilzomib therapy and suggest novel strategies that could improve desensitization regimens and reduce antibody-mediated rejection in organ transplantation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.